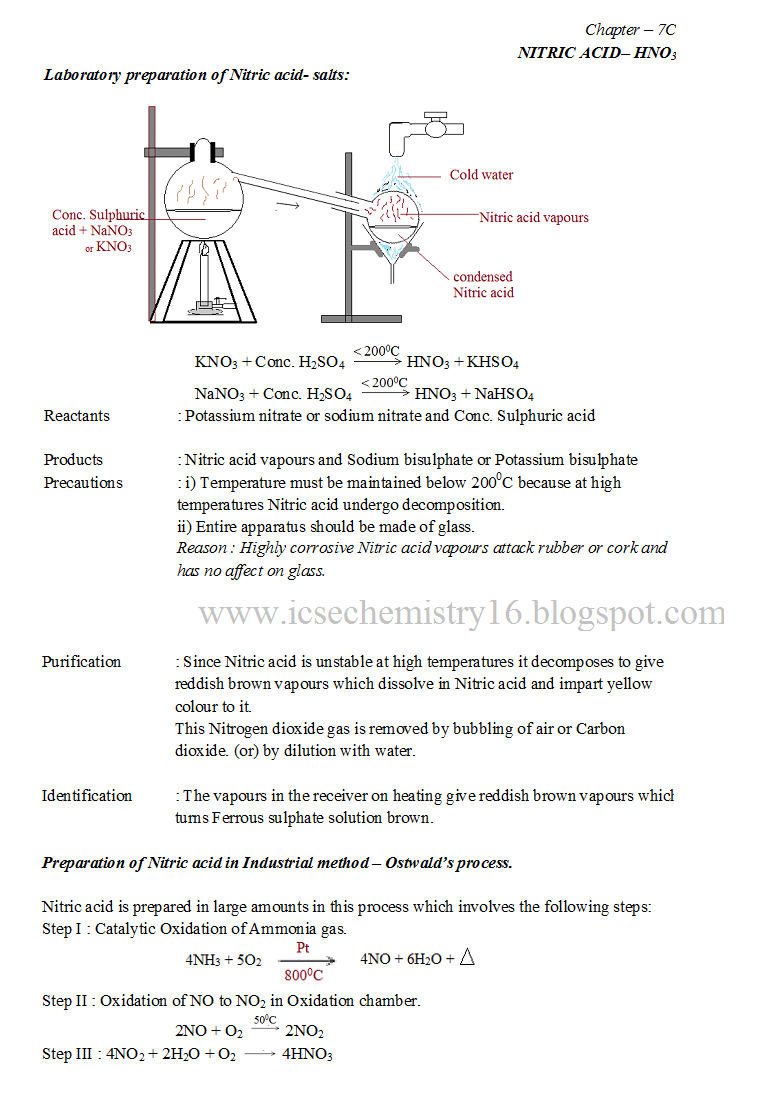
Laboratory Preparation Of Nitric Acid Balanced Equation . Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. nitric acid is prepared by the reaction of conc. In the laboratory, nitric acid (hno 3) is prepared by the action of conc. give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : (i) name a (a liquid), b( a. Preparation of hno 3 from. write a balanced chemical equation for the laboratory preparation of nitric acid. (i) kno 3 (ii) nano 3. the figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: H 2 so 4 on potassium. laboratory preparation of nitric acid: The balanced chemical equation for the laboratory preparation of nitric acid is: Answer below is the equation for the lab. (a) laboratory preparation of ethyne from calcium carbide. give balanced equations for the following:
from icsechemistry16.blogspot.com
Preparation of hno 3 from. H 2 so 4 on potassium. Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. Answer below is the equation for the lab. laboratory preparation of nitric acid: write a balanced chemical equation for the laboratory preparation of nitric acid. give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : In the laboratory, nitric acid (hno 3) is prepared by the action of conc. The balanced chemical equation for the laboratory preparation of nitric acid is: Sulphuric acid with potassium or sodium nitrate.
Nitric acid lab preparation precautions
Laboratory Preparation Of Nitric Acid Balanced Equation (i) kno 3 (ii) nano 3. nitric acid is prepared by the reaction of conc. Answer below is the equation for the lab. laboratory preparation of nitric acid: In the laboratory, nitric acid (hno 3) is prepared by the action of conc. The balanced chemical equation for the laboratory preparation of nitric acid is: give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : (i) name a (a liquid), b( a. Preparation of hno 3 from. (a) laboratory preparation of ethyne from calcium carbide. Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. Sulphuric acid with potassium or sodium nitrate. (i) kno 3 (ii) nano 3. H 2 so 4 on potassium. write a balanced chemical equation for the laboratory preparation of nitric acid. the figure given below illustrates the apparatus used in the laboratory preparation of nitric acid:
From www.doubtnut.com
Explain the following In the laboratory preparation of nitric ac Laboratory Preparation Of Nitric Acid Balanced Equation give balanced equations for the following: Preparation of hno 3 from. write a balanced chemical equation for the laboratory preparation of nitric acid. give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : (i) name a (a liquid), b( a. nitric acid is prepared by the reaction of conc.. Laboratory Preparation Of Nitric Acid Balanced Equation.
From icsechemistry16.blogspot.com
Nitric acid lab preparation precautions Laboratory Preparation Of Nitric Acid Balanced Equation (a) laboratory preparation of ethyne from calcium carbide. nitric acid is prepared by the reaction of conc. write a balanced chemical equation for the laboratory preparation of nitric acid. the figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: Preparation of hno 3 from. In the laboratory, nitric acid (hno 3) is. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.youtube.com
Preparation of nitric acid from class 12 nitric acid preparation and Laboratory Preparation Of Nitric Acid Balanced Equation give balanced equations for the following: give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. In the laboratory, nitric acid (hno 3) is prepared by the action of conc. the figure given below illustrates the. Laboratory Preparation Of Nitric Acid Balanced Equation.
From iqclasses.in
Class 10 ICSE Chemistry Board Question Chapter Nitric Acid Laboratory Preparation Of Nitric Acid Balanced Equation (i) kno 3 (ii) nano 3. Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. laboratory preparation of nitric acid: give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : In the laboratory, nitric acid (hno 3) is prepared by the action of conc. the. Laboratory Preparation Of Nitric Acid Balanced Equation.
From owlcation.com
3 Ways to Prepare Nitric Acid Owlcation Laboratory Preparation Of Nitric Acid Balanced Equation give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. give balanced equations for the following: Answer below is the equation for the lab. (i) name a (a liquid), b( a. laboratory preparation of nitric acid:. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.youtube.com
10ALab preparation of Nitric Acid YouTube Laboratory Preparation Of Nitric Acid Balanced Equation laboratory preparation of nitric acid: give balanced equations for the following: The balanced chemical equation for the laboratory preparation of nitric acid is: the figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : (i). Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.coursehero.com
[Solved] The correct net ionic equation for the reaction between nitric Laboratory Preparation Of Nitric Acid Balanced Equation Sulphuric acid with potassium or sodium nitrate. In the laboratory, nitric acid (hno 3) is prepared by the action of conc. Preparation of hno 3 from. H 2 so 4 on potassium. give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : give balanced equations for the following: write a. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.doubtnut.com
Explain the following In the laboratory preparation of nitric ac Laboratory Preparation Of Nitric Acid Balanced Equation Answer below is the equation for the lab. Preparation of hno 3 from. Sulphuric acid with potassium or sodium nitrate. (i) name a (a liquid), b( a. laboratory preparation of nitric acid: The balanced chemical equation for the laboratory preparation of nitric acid is: give balanced equations for the following: In the laboratory, nitric acid (hno 3) is. Laboratory Preparation Of Nitric Acid Balanced Equation.
From iqclasses.in
Class 10 ICSE Chemistry Mostlikely QuestionBank Chapter Nitric Acid Laboratory Preparation Of Nitric Acid Balanced Equation The balanced chemical equation for the laboratory preparation of nitric acid is: Answer below is the equation for the lab. (i) name a (a liquid), b( a. In the laboratory, nitric acid (hno 3) is prepared by the action of conc. give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : Preparation. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.youtube.com
Preparation of Nitric Acid in Laboratory Reaction HNO3 in the Lab Laboratory Preparation Of Nitric Acid Balanced Equation the figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: nitric acid is prepared by the reaction of conc. laboratory preparation of nitric acid: Preparation of hno 3 from. (a) laboratory preparation of ethyne from calcium carbide. H 2 so 4 on potassium. In the laboratory, nitric acid (hno 3) is prepared. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.vcpmaps.com
Preparation of Nitric Acid Chart at Lowest Price in Delhi Laboratory Preparation Of Nitric Acid Balanced Equation Sulphuric acid with potassium or sodium nitrate. Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. (i) kno 3 (ii) nano 3. The balanced chemical equation for the laboratory preparation of nitric acid is: give balanced equations for the following: (i) name a (a liquid), b( a. H 2 so 4 on potassium. Answer. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.istockphoto.com
Laboratory Preparation Of Nitric Acid Stock Illustration Download Laboratory Preparation Of Nitric Acid Balanced Equation write a balanced chemical equation for the laboratory preparation of nitric acid. Preparation of hno 3 from. The balanced chemical equation for the laboratory preparation of nitric acid is: (i) name a (a liquid), b( a. give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : (i) kno 3 (ii) nano. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.dbioscharts.com
CH 900 PREPARATION OF NITRIC ACID Dbios Charts Laboratory Preparation Of Nitric Acid Balanced Equation nitric acid is prepared by the reaction of conc. Answer below is the equation for the lab. give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : Preparation of hno 3 from. Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. (i) kno 3 (ii) nano. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Laboratory Preparation Of Nitric Acid Balanced Equation (i) kno 3 (ii) nano 3. H 2 so 4 on potassium. write a balanced chemical equation for the laboratory preparation of nitric acid. Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. nitric acid is prepared by the reaction of conc. (i) name a (a liquid), b( a. give a word. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Laboratory Preparation Of Nitric Acid Balanced Equation The balanced chemical equation for the laboratory preparation of nitric acid is: the figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: give balanced equations for the following: nitric acid is prepared by the reaction of conc. write a balanced chemical equation for the laboratory preparation of nitric acid. give. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.alamy.com
Laboratory preparation of nitric (V) acid fully labeled diagram Stock Laboratory Preparation Of Nitric Acid Balanced Equation (i) name a (a liquid), b( a. the figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: Answer below is the equation for the lab. In the laboratory, nitric acid (hno 3) is prepared by the action of conc. (i) kno 3 (ii) nano 3. nitric acid is prepared by the reaction of. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.youtube.com
Laboratory Preparation of Nitric AcidNITRIC ACID Class10 ICSE Laboratory Preparation Of Nitric Acid Balanced Equation In the laboratory, nitric acid (hno 3) is prepared by the action of conc. give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : Preparation of hno 3 from. the figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: write a balanced chemical equation for. Laboratory Preparation Of Nitric Acid Balanced Equation.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper Laboratory Preparation Of Nitric Acid Balanced Equation give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from : (a) laboratory preparation of ethyne from calcium carbide. give balanced equations for the following: H 2 so 4 on potassium. Kno 3 + h 2 so 4 \[\to \] khso 4 + hno 3. the figure given below illustrates the. Laboratory Preparation Of Nitric Acid Balanced Equation.